
 |
 |
 |
2. ORIGINS
2.1 Uranium Enrichment by Gaseous Diffusion
A process of uranium enrichment with the fissible isotope 235U called gaseous diffusion is used in the United States. The gaseous diffusion process requires uranium in the form of uranium hexafluoride (UF6), primarily because the compound can be used in the gas form for processing, in the liquid form for filling containers, and in the solid form for storage. At atmospheric pressure, UF6 is a solid at temperatures below 134ºF (57ºC) and a gas above this temperature. Solid UF6 is a white crystalline material resembling rock salt. Liquid UF6 is formed only at temperatures above 147ºF (64ºC) and at pressures above the atmospheric pressure (1.5 atm or 22 psi).
UF6 is highly reactive with water, forming water soluble reaction products such as uranyl fluoride (UO2F2) and hydrogen fluoride (HF), both of which are toxic. External contact with HF results in chemical burns of the skin, while exposure to airborne HF causes damage of the eyes, nose, and throat.
 |
2.2 DU Storage Sites
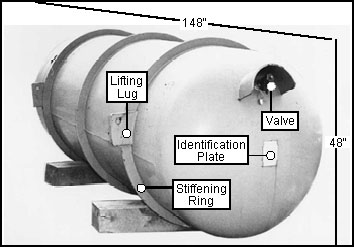
As of June 1998 [86], the US Department of Energy (DOE) owned approximately 57,800 steel cylinders of depleted uranium hexafluoride that hold a maximum of 14 short tons each (12.6 metric tons each, since 1 short ton equals 2,000 lb.), for a total mass of 734,000 metric tons. Depleted uranium alone amounts to 67.6% of this mass (the remaining 32.4% is fluorine), or 496,000 metric tons. The total radioactivity of depleted UF6 is approximately 8.6 Ci/cylinder for a total of 527,000 Ci and the a-activity 3.16 Ci/cylinder for a total of 193,000 Ci. Depleted UF6 continues to be produced at the rate about 150 cylinders (2,100 short tons or 1,900 metric tons) per year [86].
The cylinders (see Fig. 1) are stored at 3 gaseous diffusion plant sites located near Paducah, Kentucky; Portsmouth, Ohio; and Oak Ridge, Tennessee. The Oak Ridge K-25 plant was closed in 1985, while the nuclear weapons Y-12 plant remains operational. The oldest cylinders have been in storage since 1956. DOE plans to store these cylinders until the year 2020. Many of the cylinders exhibit extensive corrosion due to outdoor storage (see Fig. 2) and between 1990-92, 8 breached cylinders have been discovered.
 |
2.3 Final Disposal
DOE's preferred alternative of the ultimate disposition is to begin conversion of the UF6 inventory either to uranium oxide U3O8 (insoluble in water) or to uranium metal, while allowing for use of as much of this inventory as possible. Conversion to oxide for use or long-term storage would begin as soon as possible, with conversion to metal occurring only if uses are identified. According to [71], DOE has to commence construction by January 2004 and operate facilities at all 3 sites to treat and recycle depleted UF6 consistent with the National Environment Policy Act.
Depleted uranium hexafluoride UF6 was reprocessed into uranium tetrafluoride UF4 (known as "green salt") by the Sequoyah Fuels Corp. in Gore, Oklahoma (until 1992), as the first step in the DU conversion process to uranium metal or U3O8. At this site, 9,500 kg (21,000 lb.) of DU was found in the ground under the buildings and 7,000 kg (15,500 lb.) of DU was spilled inside the facility [24].
2.4 Other Enrichment Processes
K-1220 Centrifuge Plant Demonstration Facility has been recently built at the site of the decommissioned K-25 gaseous diffusion enrichment plant in Oak Ridge, Tennessee [37]. The new plant will test operations on equipment designs to be used in the gas centrifuge enrichment process. This process is currently used by the European uranium enrichment company Urenco in their plants through Britain, Netherlands, and Germany and also in the four Russian enrichment facilities in Ekaterinburg, Tomsk, Krasnoyarsk, and Angarsk [30].
A plant-scale test of a new uranium enrichment process called atomic vapor laser isotope separation (AVLIS) had been completed in October 1998 [80], [81] at the Lawrence Livermore National Laboratory in Livermore, California, for the United States Enrichment Corp. (USEC), the present operator of the two remaining gaseous diffusion uranium enrichment plants in the United States (Paducah in Kentucky and Portsmouth in Ohio). The new process does not require uranium in the form of UF6. After the test, the USEC initiated a search for a plant site for a commercial implementation of the AVLIS process. However, in June 1999, further development of the AVLIS process has been suspended [82].
2.5 Spent Fuel
Depleted uranium metal was also produced in the process of plutonium extraction from the spent fuel of nuclear reactors in the Rocky Flats Nuclear Weapons Plant (now Rocky Flats Environmental Technology Site) near Boulder, Colorado. In addition to the 3 natural isotopes 238U, 235U, and 234U, depleted uranium from this source also contains 0.003% of a man-made isotope 236U [41], an artificial member of the thorium decay series. This isotope decays to 232Th with a half-life of 2.40´107 years by emitting a-particles with energy 4,572 keV [2]. Therefore, depeleted uranium from the spent fuel has activity 0.15% higher and a-activity 0.5% higher compared to the byproduct of uranium enrichment (see Table 3)
 |
 |
 |
.
.
.
.
.
.
.
.