
 |
 |
 |
8. CHEMICAL TOXICITY
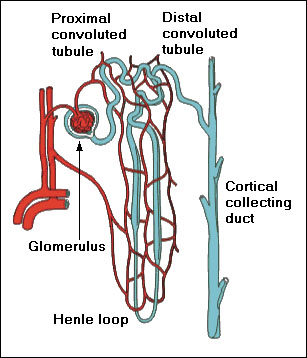 |
8.1 Uranium Effects on Kidney
Depleted uranium's radioactivity may cause severe health problems such as cancer years after exposure, but DU's chemical toxicity presents the greatest danger to health in the short term (several weeks or months) after exposure. The kidney is considered the target organ for uranium's chemical toxicity [72], [85], [86].
Kidneys filter about 160 - 200 L of blood per day, 20 - 25% of the cardiac output. The basic morphological unit of kidney is a nephron (see Fig. 9). Each kidney contains about 2 million nephrons. The most important function of the glomerulus is to serve as a sieve for plasma: Small ions and molecules such as water, sodium ions, glucose, and amino acids are filtered, while larger molecules such as proteins are not filtered. The proximal tubule actively reabsorbs 66% of sodium Na+ ions and water by means of so-called sodium-potassium-adenosine triphosphatase (Na+-K+-ATPase) pump, with chlorine Cl- ions passively following the sodium ions. Reabsorption of 70% calcium Ca2+ ions parallels reabsorption of the sodium ions. The Na+-K+-ATPase pump provides energy to reabsorb 100% of glucose and amino acids, 90% of bicarbonate (HCO3)- ions, and other electrolytes. Up to 50% of urea is reabsorbed as well. The thin descending limb of Henle loop reabsorbs water and drives up the osmotic pressure of NaCl. The ascending limb of Henle loop reabsorbs passively (in the thin limb) and actively (in the thick limb) 25% of Na+ ions, 20% of Ca2+ ions, but no water. The distal tubule and the collecting duct actively reabsorb most of the remaining Na+ and Ca2+ ions, so that over 99% of both ions is reclaimed, and a variable amount of water. The active reabsorption of sodium ions and water is tightly controlled by a variety of stimulating and supressing hormons, acting primarily at the distal tubule and collecting ducts, in order to maintain blood levels of sodium and calcium within narrow limits [60], [61], [62].
By controlling the levels of blood electrolytes (such as sodium and bicarbonate ions), kidneys maintain the acidity of blood between pH 7.35 - 7.45. When the pH value falls below this level, the condition is called acidosis, and when the pH is above, it is called alkalosis. The major effect of mild acidosis is depression of the central nervous system, disorientation, and fatigue. The major effect of mild alkalosis is hyperexcitability of the nervous system, spontaneous stimulation (spasms) of muscles, and extreme nervousness [95]. All these symptoms are among the top 10 problems reported by the Gulf War veterans [31].
Uranium in kidney acts primarily on the proximal tubule. Dissolved uranyl-carbonate complexes decompose in this acidic environment to uranyl (UO2)2+ and bicarbonate (HCO3)- ions. Like mercury, cadmium, and other heavy-metal ions, uranyl ions depress glomerular function, tubular secretion of organic anions (acidic metabolic wastes such as lactate, citrate, etc.), and reabsorption of filtered glucose and amino acids in the proximal tubule [72], [85]. When inorganic Cd, Hg, or other heavy metals are injected into the renal artery, these metal ions are instantaneously sequestered by nondiffusible macromolecules, such as plasma proteins, and become nonfilterable at the glomerulus. Dissolved uranyl ions, similar to other heavy metal ions, react with diffusible chelating compounds to form relatively stable and inert complexes. These inert complexes are then filtered and excreted in urine. Such chelating compounds have been administered to laboratory animals immediately following exposure to uranium. This chelation therapy resulted in increasing excretion of uranium in mice [79], [85].
The earlier mentioned RAND review of scientific literature on radiological and toxic effects of uranium [85] puts the overall maximum permissible concentration, i.e., concentration of metal in the kidney associated with no significant increase in the frequency of kidney malfunction, at 3 mg/kg of kidney for uranium and calls it a de facto standard. Indeed, such concentration would be reached after about 10 - 12 workweeks of exposure to air concentration of soluble uranium at the NRC occupational limit for uranium's chemical toxicity (see paragraph 8.3). In 1989, an extensive study at the Oak Ridge National Laboratory proposed to lower the maximum permissible concentration to only 300 µg/kg of kidney [94]. The saturated uranium concentration in kidney deduced from the occupational limit for soluble uranium intake by inhalation established by the Occupational Safety and Health Administration (OSHA) is 750 µg/kg (see paragraph 8.3), much lower than the RAND de facto standard. The saturated uranium concentration in kidney deduced from the minimal risk level intake of soluble uranium for general public recommended by the Agency for Toxic Substances and Disease Registry is 150 µg/kg of kidney (see paragraph 8.3), also much lower than the RAND de facto standard. The RAND de facto standard concentration of 3 mg/kg would also result in a significant radiological exposure if maintained throughout the year, 2.66 rem/year for natural and 1.52 rem/year for depleted uranium (see Table 8), which is substantially more than the RAND estimate of 400 mrem/year for natural uranium. The RAND review concludes that there is only limited evidence to suggest that even chronic exposure to natural uranium in food or drinking water, except at very high concentrations, is associated with increased mortality in humans or animals.
8.2 Uranium Excretion and Deposition in Organs
Soluble uranium (natural or depleted) which is absorbed in the blood circulation within the body is eliminated rapidly through the kidney in urine. About 67% is excreted within the first day without being deposited in any organ. Approximately 11% is initially deposited in kidney and excreted with a 15 day half-life. Most of the remaining 22% is initially deposited in the bone (up to 20%), which is the principal storage site in the body, and the rest is distributed to other organs and tissues. Uranium deposited in the bones and other organs is subsequently released back to the blood stream with at least two different half-lifes, both longer than the excretion half-life for kidney [4], [7], [51]. Similar to other heavy metals (such as mercury) causing neurotoxic problems, uranium can cross the blood-brain barrier [72], [95]. Of particular concern to women are studies with laboratory animals which found uranium in the placenta, fetus, and milk of females and in the tissues and urine of offspring fed milk from exposed females [17]. Effects ranged from low birth weight to skeletal abnormalities for doses at which the mother exhibited signs of chemical toxicity [33]. Depleted uranium was also found in semen samples from some Gulf War veterans exposed to DU [85].
8.3 Toxicological Limits on Uranium Intake
The NRC maximum occupational air concentration for soluble uranium containing less than 5% of 235U (including natural, depleted, and reactor grade enriched uranium) is 0.2 mg/m3 for a 40-hour workweek, more restrictive than the radiological limit in Table 10 because of uranium's chemical toxicity [20]. Based on about 34 m3 respirated air during this time, with 25% of the inhaled uranium dust trapped in lungs and 50% swallowed [13], this air concentration would correspond to soluble uranium intake of 5.1 mg/kg (1.7 mg/week in lungs and 3.4 mg/week in gastrointestinal (GI) tract). According to [63], only 12% of all ingested uranium (soluble and insoluble) is absorbed from intestine to blood over 6 days before the remainder is excreted in feces. Since very little insoluble uranium can enter the blood stream, it is reasonable to assume that the fraction for soluble uranium is double the value for all uranium, about 25%. Consequently, the uranium load to blood would be only 2.5 mg/week (1.7 mg/week from lungs and 0.8 mg/week from GI tract). After about 10 - 12 workweeks of exposure to the above occupational limit, 95% of the saturated uranium concentration C in kidney would be reached:
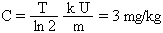
In the last formula, U = 0.36 mg/day is the average daily load of uranium to blood, k = 11% is the amount of uranium deposited in kidney, T = 15 days is the excretion half-life for the deposited uranium (see paragraph 8.2), ln 2 = 0.693, and m = 0.29 kg is the combined weight of both kidneys estimated from a 70 kg body weight and 1 : 240 ratio between the combined weight of both kidneys and the body weight [3].
Occupational limits for tungsten, lead, and uranium inhalation established by the Occupational Safety and Health Administration (OSHA) are compared in Table 13 from [13], [72]. Note that the maximum air concentration for soluble uranium is 4´ less than the NRC occupational limit and would therefore result in the saturated concentration 750 µg/kg in kidney. Soluble uranium exhibits about the same chemical toxicity as soluble lead and it is about 20´ more toxic than soluble tungsten, the alternative material for armor penetrating ammunition. The OSHA toxicological limit for the air concentration of insoluble uranium dust is superfluous, because the NRC radiological derived air concentrations for insoluble natural and depleted uranium dust are 8.3´ and 4.6´ less than this limit (see Table 10 and paragraph 7.4).
The minimal risk level for intermediate-duration ingestion by general public proposed by the Agency for Toxic Substances and Disease Registry (ATSDR) in [58] is an oral uptake of uranium at the level 1 µg/day for each kilogram of body weight, based on adverse effects observed with rats at uptakes of 1.1 mg/day/kg. For a body weight of 70 kg, this corresponds to the uranium uptake 500 µg/week into gastrointestinal (GI) tract and saturated concentration 150 µg/kg in kidney. Slightly lower tolerable uptake of 0.7 µg/day/kg is proposed in [50], based on adverse effects observed in kidneys of rabbits, corresponding to the uranium uptake 350 µg/week into GI tract and saturated concentration 100 µg/kg in kidney.
Current Environmental Protection Agency (EPA) groundwater standards at inactive uranium processing sites and the proposed standards for drinking water (see Table 14) are based on these tolerable uptakes and on the average daily water intake (see paragraph 7.4). Because of uranium's chemical toxicity, the EPA standards for water an order of magnitude lower than the NRC radiological limit in Table 12.
8.4 Gulf War Exposure to Uranium
Let us estimate the uranium concentration in kidney in Gulf War veterans after 5 days of combat. The air concentration of depleted uranium dust from explosion of a DU tank round is 24 nCi/m3 in the close proximity of the target (see paragraph 7.5), corresponding to about 20 mg/m3 of soluble and 40 mg/m3 of insoluble uranium. Assuming the average respiration rate 0.85 m3/hour, 25% of inhaled uranium dust trapped in lungs and 50% subsequently swallowed [13], this concentration leads to the soluble uranium intake of 13 mg/hour (4.3 mg/hour in lungs and 8.7 mg/hour in GI tract). Let us further assume that the US soldiers spent only 20 min/day inside or in the close proximity of the destroyed Iraqi military vehicles or bunkers, as they searched for hidden Iraqi soldiers shortly after these targets were hit by DU ammunition. The soluble uranium intake was then only 4.3 mg/day (1.4 mg/day in lungs and 2.9 mg/day in GI tract) and the uranium load to blood only 2.1 mg/day (1.4 from lungs and 0.7 mg/day from GI tract, see paragraph 8.3). Quick absorption of uranium to blood for 5 days at this rate would result in the uranium concentration C in kidney:
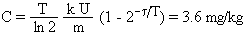
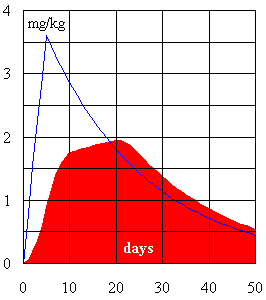 |
where t = 5 days of combat, U = 2.1 mg/day is the average daily uranium load to blood, k = 11% is the amount of uranium deposited in kidney, T = 15 days is the excretion half-life for the deposited uranium [7], ln 2 = 0.693, and m = 0.29 kg is the combined weight of both kidneys [3]. Progress of the uranium concentration in kidney according to this simple calculation is shown in Fig. 10 (blue line). However, quick absorption of the trapped and ingested uranium to blood is not a realistic assumption. When time periods of 25 and 6 days were included in the calculation [63], to allow for a slower absorption of uranium from the lungs and GI tract, the maximum uranium concentration of 2 mg/kg in kidney was reached on the 20th day and 90% of this maximum value was exceeded for 15 days beginning with day 10 (see Fig. 10, red area).
Of course, the actual air concentration of depleted uranium depended on how soon after the explosions the US troops arrived and what kind of combat activities they engaged in (such as throwing hand grenades, etc.). The resulting uranium concentration in kidney also depended on how much time they spent around the contaminated targets, but it could easily approach the value deduced from NRC occupational limit for uranium's chemical toxicity, exceed the value deduced from OSHA occupational limit for uranium inhalation by a factor of 3´, and exceed the value deduced from recommended ATSDR minimal risk level intake for general public by a factor of 17´ (see paragraph 8.3).
8.5 Chronic Exposure to Uranium
A recent study [68] on the effects of chronic ingestion of uranium in drinking water on humans found that kidney function is affected by uranium uptakes previously considered safe based on animal studies. Subjects in this study were divided into two groups: The low-exposure group (20 subjects), whose drinking water was obtained from a municipal water system and contained less than 1 µg/L of uranium, and the high-exposure group (30 subjects), whose drinking water was obtained from private drilled wells and contained 2 - 781 µg/L of uranium. These levels caused uranium intakes in the range of 0.004 - 9 µg/day per 1 kg of body weight. The exposed group excreted significantly more glucose in the urine than did the control group. Excretion of alkaline phosphatase (a marker of cell toxicity) and of b-2 microglobulin (a marker of proximal tubular function) were positively correlated with uranium intake.
The study concludes that long-term ingestion of uranium by humans may produce interference with kidney function at the elevated levels of uranium found in some groundwater supplies. The observed effects will not necessarily lead to kidney failure or overt illness, but it may be the first step leading to progressive or irreversible kidney injury [68]. Persons previously exposed to uranium may be at greater risk in the event of subsequent kidney disease than unexposed persons, since it has been observed that up to 25% renal function can be lost before problems are detected and up to 75% can be lost before onset of clear clinical symptoms [13].
8.6 Cancer Development Caused by DU
In another recent study [73], immortalized human osteoblastic (new bone formation) cells were exposed to DU-uranyl (UO2)2+ ions in vitro. Only 1 in 70,000 cell nuclei was hit by alpha particles. Nevertheless, the uranyl ions were able to transform these cells to the neoplastic (cancer) cells. The transformed cells were characterized by anchorage-independent growth, tumor formation in nude mice, high levels of oncogenes, reduced production of the tumor-suppressor protein, and elevated levels of sister chromatid exchanges.
DU-uranyl chloride (UO2Cl2) treatment resulted in a (9.6 ± 2.8)-fold increase in transformation frequency compared to untreated cells. In comparison, nickel sulfate (NiSO4) resulted in a (7.1 ± 2.1)-fold increase in transformation frequency. The implication is that the risk of cancer from internalized DU may be comparable to other biologically reactive and carcinogenic heavy metals [73].
 |
 |
 |
.
.
.
.
.
.
.
.